Embark on an enlightening journey with the acid or base indicator crossword, a captivating puzzle that unravels the intricacies of chemical reactions. Immerse yourself in a world where colors dance and solutions transform, guided by the enigmatic signals of acid-base indicators.
These remarkable substances, like alchemists of chemistry, possess the power to reveal the hidden nature of solutions, changing their hues in response to the subtle shifts in acidity or basicity. Join us as we delve into the fascinating world of acid-base indicators, exploring their types, applications, and the intriguing crossword puzzle that awaits your intellectual prowess.
Introduction

Acid-base indicators play a vital role in chemistry, particularly in acid-base titrations, by providing a visual indication of the endpoint of the reaction. A crossword puzzle is a word game that consists of a grid of squares, some of which contain letters and others that are blank.
The goal of the puzzle is to fill in the blank squares with letters to form words that fit the clues given.
Crossword puzzles can be used to teach various chemistry concepts, including acid-base indicators. By completing a crossword puzzle on acid-base indicators, students can learn about the different types of indicators, their properties, and their applications.
Types of Acid-Base Indicators: Acid Or Base Indicator Crossword
Acid-base indicators are substances that undergo a change in color when the pH of a solution changes. They are commonly used in titrations to determine the endpoint of a reaction.
There are many different types of acid-base indicators, each with its own unique color change. Some of the most common indicators include:
- Phenolphthalein: Phenolphthalein is a colorless indicator that turns pink in basic solutions.
- Methyl orange: Methyl orange is a yellow indicator that turns red in acidic solutions.
- Litmus: Litmus is a purple indicator that turns red in acidic solutions and blue in basic solutions.
The color change of an acid-base indicator is caused by a chemical reaction. In the case of phenolphthalein, the indicator is a weak acid that dissociates in water to form hydrogen ions (H+) and phenolphthalein ions (C20H14O4). In acidic solutions, the concentration of H+ ions is high, which causes the phenolphthalein ions to protonate and form the colorless H2C20H14O4 molecule.
In basic solutions, the concentration of H+ ions is low, which causes the H2C20H14O4 molecule to deprotonate and form the pink C20H14O42- ion.
The color change of other acid-base indicators is caused by similar chemical reactions.
Applications of Acid-Base Indicators

Acid-base indicators are widely employed in analytical chemistry for various purposes, primarily involving the determination of pH and acid-base titrations.
Determination of pH
Indicators are used to determine the pH of solutions by undergoing a visible color change over a specific pH range. The color change is due to the protonation or deprotonation of the indicator molecule, resulting in a shift in its absorption spectrum.
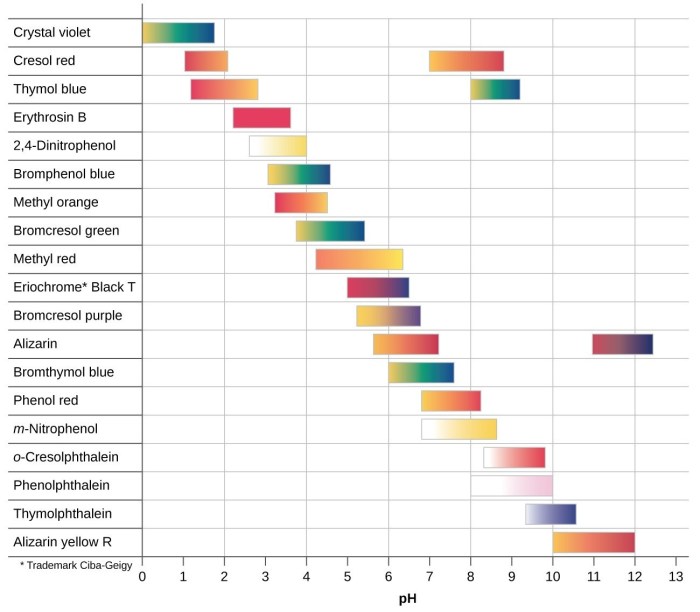
The pH of a solution can be estimated by comparing the color of the indicator to a color chart or using a pH meter. The pH meter provides a more accurate measurement, but indicators offer a quick and convenient method for approximate pH determination.
Acid-Base Titrations, Acid or base indicator crossword
Acid-base titrations involve the gradual addition of a known concentration of acid or base to a solution of unknown concentration until the equivalence point is reached. Indicators play a crucial role in signaling the equivalence point by undergoing a sharp color change.
The equivalence point is the point at which the moles of acid added are equal to the moles of base present in the solution. At this point, the indicator changes color, indicating the completion of the titration.
The choice of indicator for a particular titration depends on the pH range of the equivalence point and the desired color change. A suitable indicator should have a color change that is easily visible and occurs close to the equivalence point.
Acid-Base Indicator Crossword
Acid-base indicators are substances that change color in response to changes in the pH of a solution. They are used in a variety of applications, including titrations, pH measurements, and the identification of unknown solutions.
This crossword puzzle includes clues related to acid-base indicators. The answers to the crossword puzzle are provided in a table below. The crossword puzzle can be used to reinforce understanding of acid-base indicators by providing a fun and interactive way to learn about these important substances.
Crossword Puzzle
Across
- A substance that changes color in response to changes in the pH of a solution (8 letters)
- The pH range over which an acid-base indicator changes color (7 letters)
- A type of acid-base indicator that is used in titrations (6 letters)
- A type of acid-base indicator that is used in pH measurements (5 letters)
- A type of acid-base indicator that is used to identify unknown solutions (4 letters)
Down
- The point at which an acid-base indicator changes color (9 letters)
- The process of using an acid-base indicator to determine the pH of a solution (7 letters)
- A device used to measure the pH of a solution (5 letters)
Answers to the Crossword Puzzle
| Across | Down |
|---|---|
| INDICATOR | ENDPOINT |
| TRANSITION INTERVAL | TITRATION |
| PHENOLPHTHALEIN | pH METER |
| METHYL ORANGE | |
| LITMUS |
Conclusion
Acid-base indicators are indispensable tools in chemistry, providing a convenient and visual method for determining the acidity or basicity of a solution. They play a crucial role in various applications, including analytical chemistry, titrations, and the study of equilibrium reactions.The
key points discussed in this article include:* The different types of acid-base indicators and their mechanisms of action
- The importance of pH in understanding the behavior of acid-base indicators
- The applications of acid-base indicators in chemistry and beyond
By understanding the principles and applications of acid-base indicators, chemists can gain valuable insights into the behavior of chemical systems and make informed decisions in their research and practical work.
Further Exploration
For further exploration of this topic, readers are encouraged to consult the following resources:* [Book] Acid-Base Indicators by Hammett and Deyrup
- [Website] Acid-Base Indicators from the University of California, Berkeley
- [Journal Article] The Role of Acid-Base Indicators in Analytical Chemistry by Smith and March
FAQ Insights
What is the purpose of acid-base indicators?
Acid-base indicators are substances that change color in response to changes in acidity or basicity, providing a visual cue to determine the pH of a solution.
How do acid-base indicators work?
Acid-base indicators undergo chemical reactions that alter their molecular structure, resulting in a change in color. This color change is specific to the pH range of the indicator.
What are some common applications of acid-base indicators?
Acid-base indicators are widely used in analytical chemistry, including pH determination, acid-base titrations, and as visual aids in various chemical reactions.