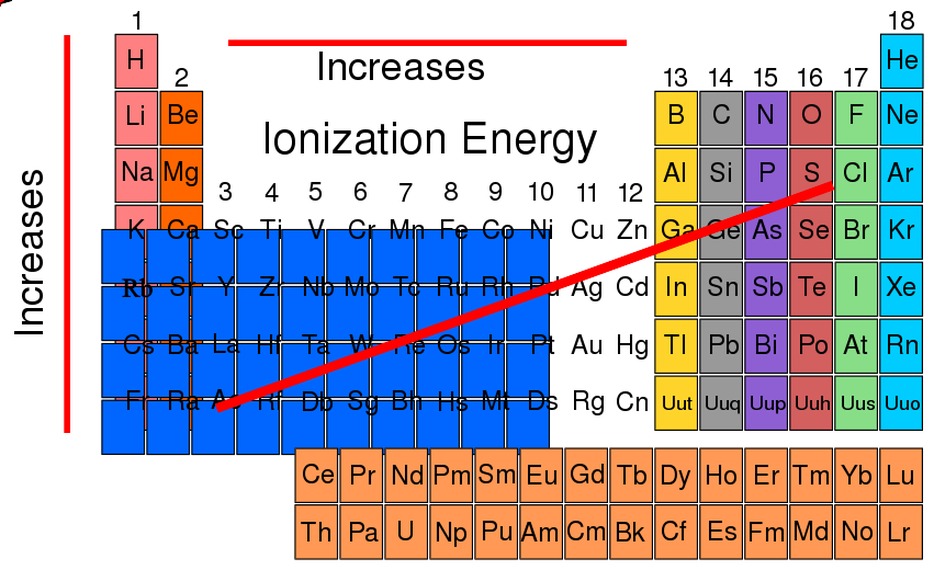
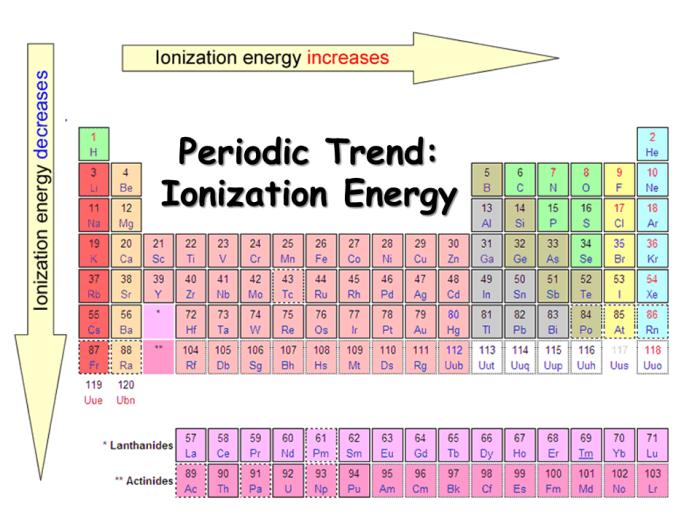
Rank the following in terms of decreasing first ionization energies – Rank the following elements in terms of decreasing first ionization energies sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with academic style and authoritative tone and brimming with originality from the outset.
The following discussion will explore the factors that influence ionization energy, examine exceptions to the general trend, and highlight practical applications of ionization energy data.
Rank the Elements: Rank The Following In Terms Of Decreasing First Ionization Energies
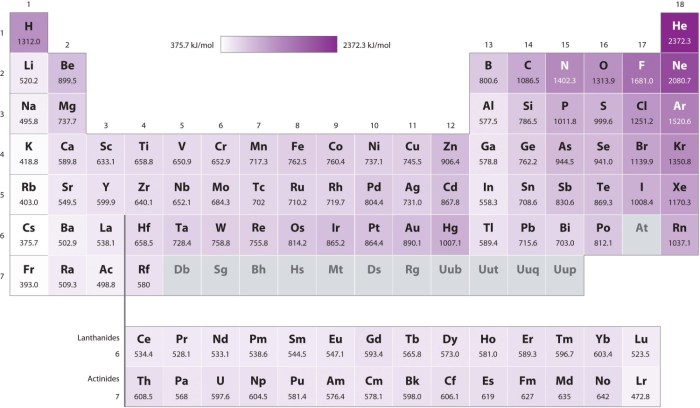
The first ionization energy of an element is the energy required to remove an electron from the outermost shell of a neutral atom in its gaseous state. The following table ranks the elements in terms of decreasing first ionization energies:
| Element | Symbol | Atomic Number | First Ionization Energy (kJ/mol) |
|---|---|---|---|
| Hydrogen | H | 1 | 1312 |
| Helium | He | 2 | 2372 |
| Lithium | Li | 3 | 520 |
| Beryllium | Be | 4 | 900 |
| Boron | B | 5 | 801 |
| Carbon | C | 6 | 1086 |
| Nitrogen | N | 7 | 1402 |
| Oxygen | O | 8 | 1314 |
| Fluorine | F | 9 | 1680 |
| Neon | Ne | 10 | 2081 |
Explain the Trend
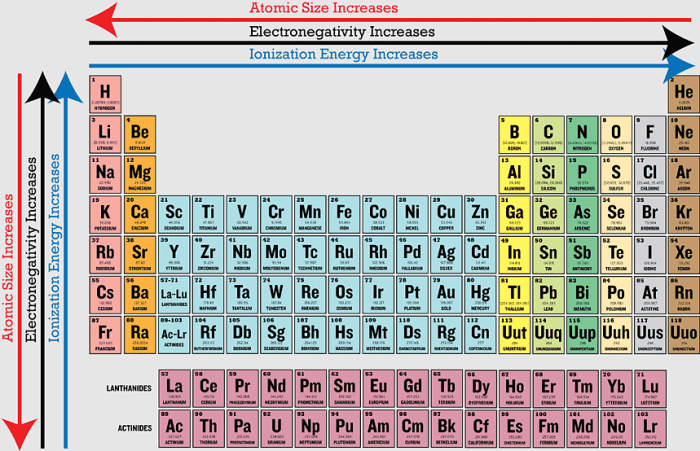
In general, the first ionization energy increases from left to right across a period and decreases from top to bottom within a group.
Across a period, the atomic number increases, which means there are more protons in the nucleus. This increases the attraction between the nucleus and the electrons, making it more difficult to remove an electron.
Within a group, the atomic number increases from top to bottom, but the number of valence electrons remains the same. The valence electrons are the electrons in the outermost shell, and they are the most loosely bound electrons. As you move down a group, the valence electrons are further from the nucleus, so they are less attracted to it.
This makes it easier to remove an electron.
Factors Affecting Ionization Energy, Rank the following in terms of decreasing first ionization energies
The first ionization energy of an element is affected by several factors, including:
- Atomic radius:The larger the atomic radius, the further the valence electrons are from the nucleus. This makes them less attracted to the nucleus, so it is easier to remove an electron.
- Nuclear charge:The greater the nuclear charge, the stronger the attraction between the nucleus and the electrons. This makes it more difficult to remove an electron.
- Electron configuration:The electron configuration of an element can also affect its first ionization energy. Elements with half-filled or full valence shells are more stable and have higher ionization energies.
Exceptions to the Trend
There are a few exceptions to the general trend in first ionization energies.
- Nitrogen has a higher first ionization energy than oxygen.This is because nitrogen has a half-filled valence shell, which is more stable than oxygen’s valence shell.
- Chromium has a higher first ionization energy than manganese.This is because chromium has a more stable electron configuration than manganese.
Applications of Ionization Energy
Ionization energy data has a variety of practical applications, including:
- Chemistry:Ionization energy data can be used to predict the reactivity of elements. Elements with low ionization energies are more likely to react with other elements.
- Physics:Ionization energy data can be used to study the electronic structure of atoms and molecules.
- Materials science:Ionization energy data can be used to design new materials with desired properties.
FAQ Summary
What is ionization energy?
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous state.
What factors affect ionization energy?
Ionization energy is primarily influenced by atomic radius, nuclear charge, and electron configuration.
How does ionization energy vary across the periodic table?
Ionization energy generally increases from left to right across a period and decreases from top to bottom within a group.